The Food and Drug Administration (FDA) is providing information and recommendations regarding safety concerns with the recalled Riata leads. These leads have an increased risk of premature insulation failure that can impact the lead’s ability to function properly.
AUDIENCE: Cardiology, Radiology, Surgery, Family Care Physicians
ISSUE: The Food and Drug Administration (FDA) is providing information and recommendations regarding safety concerns with the recalled Riata leads. These leads have an increased risk of premature insulation failure that can impact the lead’s ability to function properly.
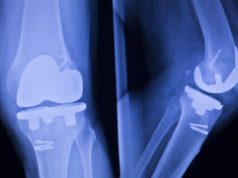
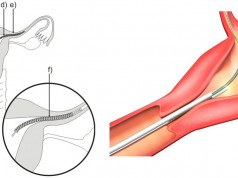
Many factors contribute to the lifespan of an ICD lead, including the age and activity level of the patient. On average, an ICD lead is expected to last at least 10 years. The FDA is aware of an increase in frequency of reported Riata insulation failures, beginning approximately four years after implant. Insulation failure may cause some of the electrical conductors inside Riata leads to move within (migrate), or move entirely outside (externalize), the outer lead insulation. These changes may be detectable on X-ray or fluoroscopic imaging.
Lead insulation failure may cause the ICD lead to malfunction. ICD lead malfunction may cause abnormal sensing or pacing, or delivery of inappropriate or no shock therapy, which could result in serious adverse events, including death.
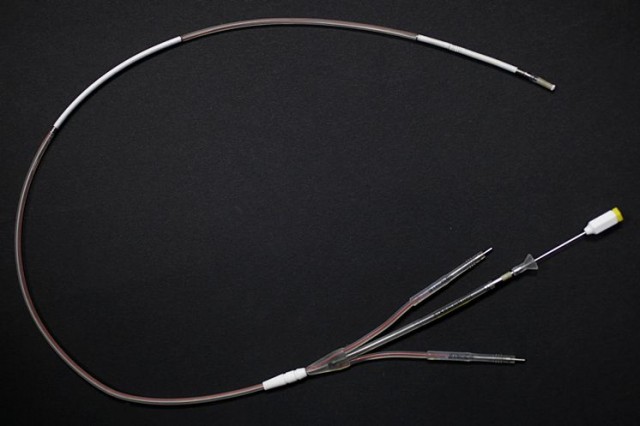
BACKGROUND: Riata and Riata ST leads connect an implantable cardioverter defibrillator (ICD) to the heart in order to monitor heart rhythms. ICD’s can detect life-threatening heart rhythms and deliver an electrical shock from the ICD through the lead to the heart. ICD leads typically have layers of insulation that protect electrical conductor wires inside the lead.
Riata’s manufacturer, St. Jude Medical Inc., recalled these leads on Nov. 28, 2011, due to premature erosion of the insulation around the electrical conductor wires, known as insulation failure. St. Jude Medical stopped selling these leads in late 2010 but more than 227,000 Riata leads had been distributed worldwide. According to St. Jude Medical, as of 2011, approximately 79,000 Riata leads remained implanted in patients in the United States.
If you suspect that an ICD lead has premature insulation failure, externalized or migrated conductors, electrical malfunction, or other abnormal function, the FDA encourages you to file a voluntary report to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report Online: www.fda.gov/MedWatch/report.htm
- Or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178