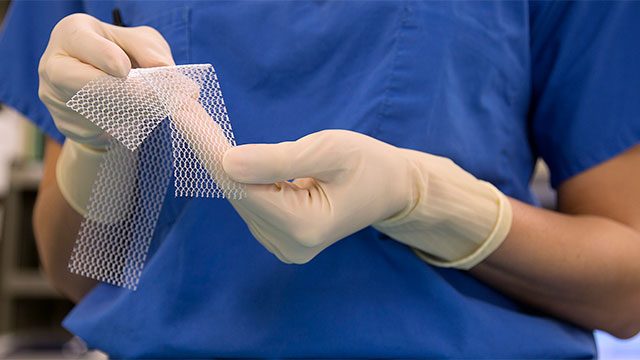
The use of Physiomesh has been shown to increase the risk of revision surgeries and hernia recurrence. The product was removed from the market in May of 2016, but many people who’ve already had surgery are still suffering from serious complications.
A hernia occurs when an organ, intestine, or tissue pushes through a hole in the surrounding muscle or connective tissue. A hernia mesh (such as the Physiomesh) is a device which is implanted in order to keep the tissue in place.
Ethicon’s Physiomesh®, which had been in the market for a little over five years, was shown to cause post-surgical complications. Those suffering complications due to the mesh device may be able to recover significant compensation through a product liability claim.
What is Physiomesh?
Similar to the transvaginal mesh, Physiomesh is made from non-absorbable plastic called polypropylene, which is flexible and woven onto a special pattern. This device was in the past used to repair an abdominal hernia known as a ventral hernia. The surgery involves reinforcing the abdominal wall so that the hernia doesn’t recur or reopen. It’s estimated that over 100,000 to 150,000 such operations are carried out in the US every year.
One big issue with this kind of device is that it may migrate and damage surrounding organs and tissue. In fact, it’s not uncommon for Physiomesh to cause fistula, internal bleeding, intestinal blockage, and infection.
Here are other complications caused by Physiomesh:
- Formation of scar tissue which sticks together (also known as adhesion)
- Hernia recurrence
- Mesh shrinkage
- Excruciating pain
- Surgical site fluid buildup
“Market Withdrawal” Issued
In May of this year, Ethicon, a Johnson & Johnson subsidiary issued a Field Safety Notice alerting patients as well as customers about the high rates of complications associated with Physiomesh, issuing an official “market withdrawal” of its mesh product.
While there is no class action lawsuit brought forward by Physiomesh users, the number of complaints is growing and may lead to multidistrict litigation. The best thing to do at the moment is to contact a product liability attorney in your area for a free consultation into your legal options.