IVC Filters Can Malfunction, Fracture, Migrate, and May Damage Veins
Many patients take blood thinners in order to prevent blood clots from migrating to the lungs. Unfortunately, there are some people who are unable to take these medications. In these cases, it is necessary for a surgeon to implant a special device to take care of the function the drugs normally perform.
The Procedure and Complications
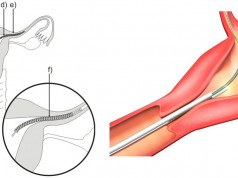
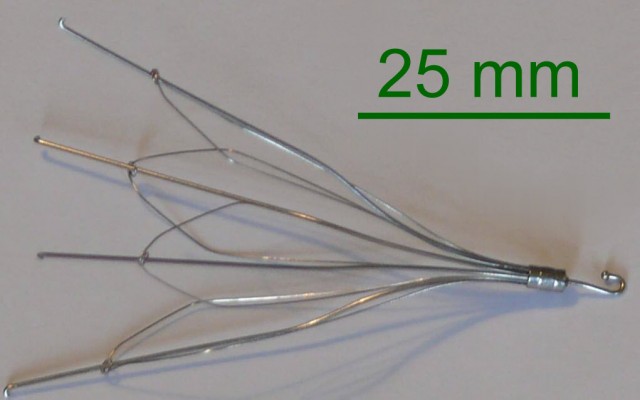
When a patient is unable to tolerate blood thinners (anticoagulants) to prevent any blood clots from migrating to the lungs, it becomes necessary for a surgeon to implant a special retrievable device in the veins of those patients. This device is called an inferior vena cava filter or IVC. If the device works properly, it will capture the clots that are present in the bloodstream, and over time the clots will dispel.
According to the U.S. Food & Drug Administration, it has received hundreds of unfavorable reports that focus on complications such as organs that have become punctured, and the migration of IVC filters to other parts of the body.
The FDA issued a warning in 2010 about the dangers of IVC filters, and recommended their removal as soon as the patient’s risk of blood clots had been alleviated. In 2014, they updated their safety communication to state that the devices should only remain in place for 29 to 54 days, but for some patients this warning was too late.
Negligent Manufacturers
Two of the primary IVC filter manufacturers targeted in IVC filter lawsuits are C.R. Bard and Cook Medical. Some of the problems associated with the devices include fractures of the device, migration, damage to the vein or organ, and even death. Five of the products that have been sources of problems and named in lawsuits include:
- The Bard Recovery filter
- The Bard G2 filter
- The Bard G2 Express filter
- The Cook Gunther Tulip filter
- The Cook Celect filter
Some of the claims filed against the manufacturers include defects in the design, failing to warn patients of the possible dangers, negligence, breach of implied warranty, defects in manufacturing, and negligent misrepresentation.
Side Effects of IVC Filter Implantation
Although the IVC filter can be beneficial for those at risk for blood clots who can’t take blood thinners, there are several potential side effects.
- Device fracture
- Migration of the filter
- Chest pain
- Perforation of the lungs, vena cava, or heart
- Excess fluid surrounding the heart
- Deep vein thrombosis of a lower limb
- Embolization of the filter
- Compression of the heart as a result of excess fluid around the heart
One other safety factor that has not been previously mentioned is that a doctor may be unable to remove the device in case of failure or health risks.
Statistics and Safety Warnings by the FDA
In August of 2010, after they had received 921 reports of adverse events that involved IVC filters, the FDA issued a safety communication. They had some concerns that temporary filters were remaining implanted even after the patients were no longer at risk of pulmonary embolism. Some of the information included in the reports was as follows:
- 328 device migrations
- 56 cases of fractures of the filter
- 70 reports of the vena cava perforation
- 146 cases that involved the filter migrating to the bloodstream
If you or a loved one has had problems with an IVC filter, you should contact a lawyer and request a free over-the-phone consultation. The Dallas IVC filter attorneys at Rasansky Law Firm (1-877-659-2580) can look into the facts of your case and help you decide the best course of action free of charge.