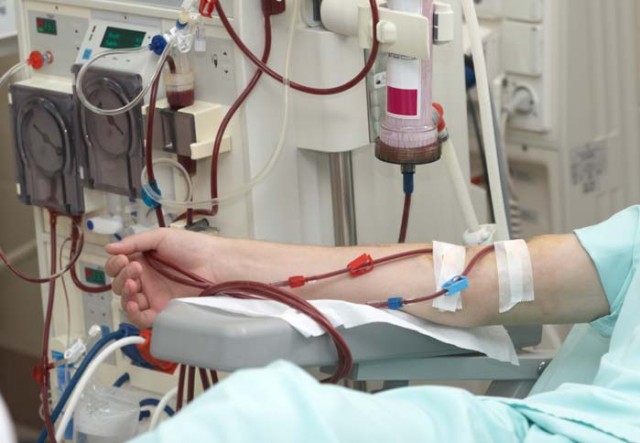
The Food and Drug Administration (FDA) has issued a recall of GranuFlo and NaturaLyte acid concentrates used during hemodialysis treatment. These unsafe drugs can cause sudden death, heart attack, cardiac arrest and other severe and fatal problems. GranuFlow is the most-widely prescribed dry acid product in the dialysis industry today. The recall occurred after an internal memo sent by Fresenius Medical Care (FMC), the manufacturer of GranuFlo and NaturaLyte, was leaked to the FDA.
Fresenius Medical Care sent this warning to their own dialysis centers but failed to send notification to thousands of physicians at dialysis clinics not owned by FMC. The manufacturer became aware of the frightning number of dialysis treatment heart problems since 2010, yet again failed to notify the FDA and did not issue a warning to clinics that were using these products.
According to the U.S. Food and Drug Administration in unsafe drug recall document released Mar 29, 2012, the recall of GranuFlo and NaruralLyte is listed as a Class 1 Recall. Class 1 recalls are the most severe form of recall issued by the FDA and occur in situations where there is reasonable probability that the use of or exposure to a product will cause serious adverse health consequences and death. The manufacturer is cautioning clinicians to be aware of the concentration of acetate or sodium diacetate (acetic acid plus acetate) contained in Fresenius’ Naturalyte Liquid and Granuflo Dry Acid Concentrate. Inappropriate prescription of these products can lead to a high serum bicarbonate level in patients undergoing hemodialysis. This may contribute to metabolic alkalosis, which is a significant risk factor associated with low blood pressure, hypokalemia, hypoxemia, hypercapnia and cardiac arrhythmia, which, if not appropriately treated, may culminate in cardiopulmonary arrest. This product may cause serious adverse health consequences, including death.
As a result of hemodialysis involving Granuflo or NaturaLyte, individuals may be entitled to financial compensation through a Fresenius dialysis lawsuit if they suffered any of the following problems during dialysis treatment or within 48 hours after:
- Heart Attack
- Cardiopulmonary Arrest
- Stroke
- Sudden Death
- Catastrophic Heart Injury
Attorneys representing injured victims and their families allege that the company was attempting to hide, mislead and obscure information about the risks in an attempt to maintain market share, placing their desire for profits ahead of the safety of dialysis patients.
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these products to The FDA Safety Information and Adverse Event Reporting Program. If you or a loved one experienced cardiac arrest, stroke or other serious side effects after receiving hemodialysis treatment with GranuFlo or NaturaLyte, contact a personal injury attorney with experience in dialysis injuries today.