Shoulder replacement surgery is often extremely helpful to those with bone and cartilage damage caused by issues such as erosive arthritis. Unfortunately, some shoulder replacement systems also have very serious health risks.
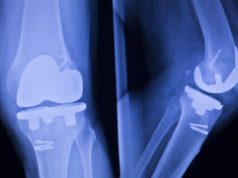
Over 50,000 people undergo reverse shoulder replacement surgery every year in an attempt to restore mobility to their arm as well as eliminate or lessen joint pain. This kind of surgery is invasive, and it involves installing steel implants into one’s shoulder joint.
Zimmer Biomet is one of the most-commonly used shoulder replacement systems in the United States, but has recently come under fire due to the product’s failure rate.
Why Shoulder Implants Are Important
Shoulder arthritis develops over time, and it can be painful and disabling to live with. When all else fails, doctors may recommend a full shoulder replacement system, which usually uses other muscles found within the rotator cuff for support.
Patients who receive this kind of implant are looking to increase their range of motion as well as get rid of the pain they have lived with for numerous years. This can markedly improve their quality of life going forward, helping them to get back to things they used to enjoy such as playing golf, painting, woodwork etc.
Zimmer Biomet’s History
Biomet manufactured over 3,000 of these devices before being acquired by Zimmer back in 2015 for $14 billion. However, the new company, Zimmer Biomet issued an “Urgent Medical Device Recall Notice” on December 20th, 2016, asking all medical professionals to quarantine stock immediately.
This recall notice was issued when it was discovered that the device was fracturing at a much higher rate than was stated in its original labeling.
On top of that, the company was only required to show that the device worked in a similar manner as other devices in the market in order to get approval from the FDA, which many have construed as negligent and careless.
Here are some of the most-serious complications associated with the Zimmer Biomet Shoulder Replacement System:
- Infections
- Loss of use of the shoulder
- Chronic, unremitting pain
- Weakness and instability
- Bone loss
- Tissue damage
- Wrongful Death
One study published in the Journal of Shoulder and Elbow Surgery revealed that individuals whose shoulder replacements failed were up to 6 times more likely to die within a month’s time, when compared to the general populace.
FDA Recalls Biomet Shoulder Device
In February of 2017, the FDA announced that it had designated Biomet’s shoulder implant notice as a Class I recall, urging all affected parties to be on the lookout for an increased risk of fracture, prompting revision surgery.
One such failure occurred in 2014 when a man who received the device back in 2009 (and again in 2010) had both implants fail within just one year of each other. He received $350,000 as compensation, and opened the door for other victims to come forward.
Compensation for Zimmer Biomet Shoulder Implant Failure
Medical device implants are prone to failure and complications if not done meticulously. However, the manufacturers of these devices are also held to a very high standard by the law when it comes to making sure they are safe.
If it can be shown that they failed in the regard, they might be held liable for any damages should a person become injured due to the device.
These kinds of cases can involve considerable damages, meaning that victims may be owed significant compensation due to the pain and suffering involved, as well as the need for revision surgery and physical rehabilitation.