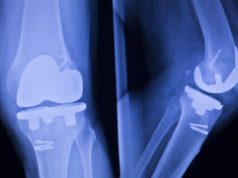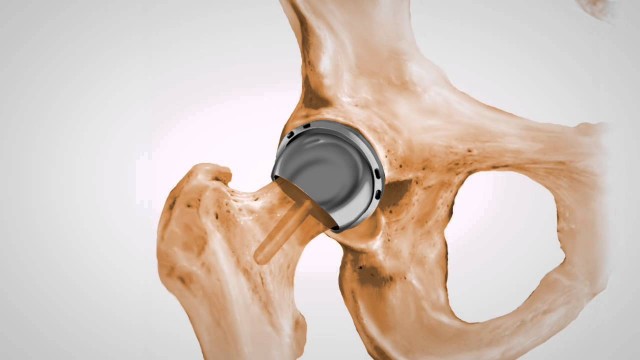
Stryker Orthopaedics has recalled two modular neck stem models for hip implants because of the risk of corrosion and wear, which can cause tissue damage. The devices are the modular-neck hip stems ABG II and Rejuvenate. The risks posed by corrosion can lead to hip implant failure and serious pain.
Voluntary Hip Implant Recall by Stryker
Stryker has voluntarily recalled its Rejuvenate and ABG II modular-neck stems. Stryker Orthopaedics’ decision to voluntarily remove Rejuvenate and ABG II modular-neck stems and terminate global distribution of these products comes after continued post-market surveillance. The post-market surveillance data may be predictive of a trend. “Following this action, we will work with the medical community to better understand this matter as we continue to evaluate the data,” said Simpson.
Stryker has notified healthcare professionals and regulatory bodies of this voluntary recall. Patients who received a Rejuvenate Modular or ABG II modular-neck stem are encouraged to contact their surgeon. Patients uncertain if they have one of these products implanted should contact their surgeon or consult their medical records.
Adverse Indications
Pain and swelling at the implant site may indicate adverse tissue reactions caused by the hip implant devices. If you have pain and swelling, you should contact your surgeon immediately.
The neck stems contain metal-on-metal components. These components are susceptible to corrosion and to abrading against one another. Wear and corrosion at the modular-neck junction can cause metal debris and ions to be released into the joint area. The presence of these materials in your body can lead to adverse tissue reactions, including:
- Metallosis, inflammation around metal particles
- Necrosis, death of the tissue
- Osteolysis, which leads to bone loss
- Severe pain
- Allergic reaction for people with metal sensitivity
Adverse tissue reactions and complications caused by corrosion can lead to premature hip implant failure and the necessity for revision surgery.
Stryker decided to recall the modular-neck stems based on post-market data, according to a press release on the FDA website. This data showed that a percentage of patients had to have revision surgeries, possibly due to corrosion and wear at the modular-neck junction.
Stryker Hip Recall Attorneys
You may be able to file a lawsuit seeking compensation for your medical expenses, pain and suffering, lost wages and more. THe attorneys at Rasansky Law Firm will be happy to answer all of your questions and inform you of your legal rights and options. Simply use the contact form on this page to get answers now.