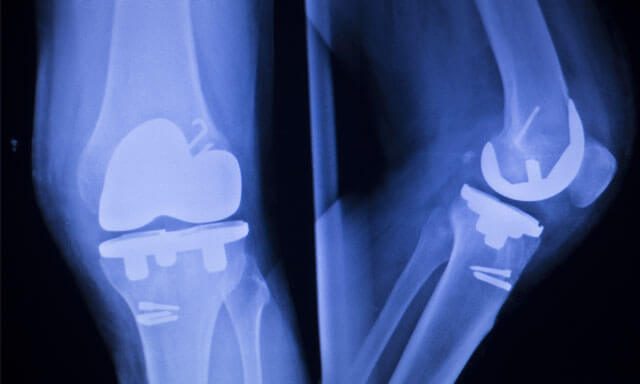
The Attune Knee Replacement System, manufactured by DePuy Orthopedics (a J&J subsidiary) has been in the news lately due to its propensity to fail.
The DePuy Attune Knee Replacement System received FDA approval in 2010, and was said to offer a wider range of motion as well as better stability when compared to other knee replacement devices in the market.
However, many individuals who received the Attune Knee replacement soon found out that the device was prone to bonding issues as well as the wearing down of components, exposing them to tibial loosening, bone loss, extreme pain and the need for revision surgery.
DePuy Knee Implant Studies
Last year, the well-respected Journal of Knee Surgery published a damning report highlighting the unusually-high rate of early failure of tibial component in the Attune Total Knee Arthroplasty System.
The report’s author states that there has been a high rate of debonding of the tibial implant cement interface. In plain English, surgeons were seeing cases where the cement joining implant parts together was dissolving, causing the loosening of tibial plates and implant failure.
A number of patients were presenting with limited range of movement as well as excruciating pain, infections and a host of complications shortly after having the knee implant installed.
How Long Should a Knee Implant Last?
As a rule of thumb, artificial joints can last for anywhere from 10 to 15 years. However, the DePuy Attune system could fail after a few months of implantation, something that is not only unacceptable, but also dangerous on account of the adverse health effects transplant recipients may experience.
Here are some of the signs that point to DePuy knee implant failure:
- Localized Pain – After surgery, it is natural to experience pain as the body adjusts to the new device. However, pain that is persistent and intractable usually points to something that’s not right with the implant. This pain presents as a throbbing or sharp knee pain even when one is not walking or using their knee while standing.
- Swelling – Loose components may cause tissue irritation, leading to an inflamed knee. This then leads to the buildup of fluid and subsequent knee swelling.
- Knee Instability – When the implant fails and components come loose, the use of said knee will be next impossible due to component misalignment. Shaking, wobbling and being unable to move one’s knee in different directions is one of the signs of a failed DePuy knee implant.
Knee Implant Failure – What to Do
It is important that you see a doctor as soon as you notice any of these symptoms. Failure to do so may lead to systemic infections that can quickly turn fatal if not treated in a timely manner.
In addition, you may form a fibrous scar tissue or suffer from necrosis, both of which will make it hard for your body to accept another implant or experience comfort when another one is finally implanted.
Product Liability Lawsuits for DePuy Knee Implant Devices
These kinds of medical device failures ultimately point to negligence on behalf of the manufacturer, as well as a failure to mitigate damages by warning the general public.
It can be argued that DePuy failed in its role to ensure that its products are safe, opening the company up to product liability claims filed by those who’ve suffered as a result.
If you’ve been affected by the DePuy knee implant debacle, you may be entitled to compensation. For more information, speak with a personal injury attorney in your area about the possibility of seeking compensation through a product liability claim or lawsuit.