Essure® Birth Control Devices Linked to Migration & Other Serious Health Issues
Reproductive health is a big ticket issue for women. The ability to choose whether or not to have children lies in their hands at all times, and rightfully so. There comes a time when an individual decides to no longer have children; in such cases, there are medical options that allow them the opportunity to make changes to their biology in order to permanently effect this change.
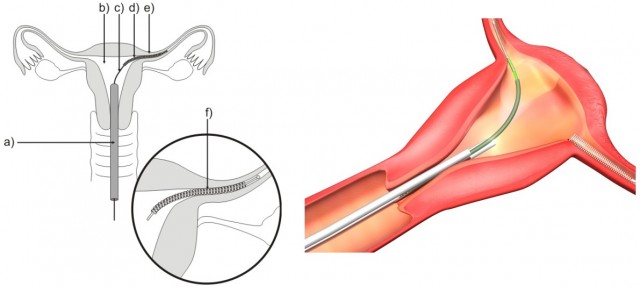
Essure® is a device marketed and sold by Bayer, a well-known pharmaceutical company which is known for a slew of drugs targeting common conditions such as heart attacks and stroke. The Essure birth control device is essentially a metal coil that is placed in a woman’s cervix with two pieces of wire in each fallopian tube.
The device then goes to work in the next couple of months, blocking these tubes by forming tissue around the wire. This means that any eggs that the woman produces for fertilization won’t get the chance of making it to the cervix wall, effectively making pregnancy impossible.
Essure Testing Flawed
According to records, Essure underwent stringent testing involving thousands of women. In one instance, 269 women were implanted with the coil, with more than 1 in 10 of these reporting serious side effects such as bleeding, back pain, painful intercourse, and painful menstrual periods in the first 3 months. In another trial which involved 558 women, a substantial number of women reported perforated fallopian tubes, expulsion of the coil, and reports of the coil ending up in different parts of the body (migration).
FDA Implicated
In what could be termed as negligence and lack of foresight, the FDA disregarded the results of the two trials above and asked that more trials and further monitoring be carried out for an additional four years. This allegedly wasn’t fully carried out according to the wishes of the regulatory body since only 171 women were monitored during this 4 year period.
A few years ago, it was accepted that due to the fact Essure was introduced into the market by way of testing via the FDA’s approval, Bayer was shielded from litigation. However, a landmark case that was heard in July of 2015 set a precedent that would allow other women to come forward and seek justice for the injuries, pain, and suffering they had to live with after the implantation of this unsafe medical device.
On February 29th, 2016, the FDA stated that it will require Bayer AG to “undertake new safety studies on its permanent birth-control device Essure,” and recommended a “black box” warning label regarding the medical device’s potentially-serious side effects, plus a risks checklist for doctors to discuss with patients. Unfortunately, they decided to allow Essure to stay on the market while new studies are undertaken.
On April 9th, 2018, The FDA announced that only women who read and sign a brochure about the risks of the device will be able to receive the Essure implant. This new requirement comes about two years after the FDA required Bayer to undergo new safety studies related to the nickel-titanium implant.
Some of the serious side-effects associated with this implant include:
- Heavy and irregular menstrual periods
- Weight gain and fluctuation
- Internal breakage and migration of the device
- Chronic pelvic and back pain
- Depression
- Extreme fatigue
- Allergic reactions to the nickel in the coil
- Suicidal thoughts
- Systemic tissue inflammation
The landmark case filed this year was brought by a South Carolina woman who had to have her right fallopian tube removed due to the amount of damage the device was alleged to have caused. This is just one of thousands of cases across the country filed due to the negligent actions of a drug company that did not carry out due diligence before launching a medical device.
Injured by Essure? Get Legal Help Today!
If you or someone you know suffered complications as a result of an Essure birth control implant, you have a right to seek legal help. Rasansky Law Firm has more than two decades of medical device injury litigation experience, and we invite you to get in touch with us (toll-free) by calling 1-877-659-2580 for a free consultation into a potential Essure lawsuit.