The Toratec Corporation’s HeartMate II Left Ventricular Assist System (LVAS) has been recalled effective February 23, 2012. If you have been implanted with one of these devices, you should have already been notified by the manufacturer or the physician who installed it. This recall affects LVAS devices with the model numbers 10393, 104692, 104911, and 104912. They were manufactured between February of 2010 and February of 2012, according to a press release by the FDA.
Serious Issues – Class I Recall
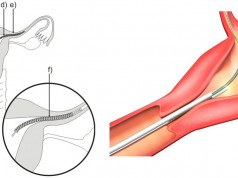
These devices are used in heart transplant candidates as a bridge to transplantation. They are also used in patients with specific types of left ventricular failure who cannot receive a heart transplant. The function of the device is to deliver blood from the left ventricle to the rest of the body. It can be used at home and is sometimes used to help patients being transported to the hospital.
The device is shown to have a problem involving the outflow graft kinking or deforming. This can end up reducing blood flow, causing a thrombosis or causing a perforation of the outflow graft. The device has also been shown to have sharp edges that could cause cutting of the outflow graft.
This defect can result in the death of the patient, so it’s important that the issue is handled immediately.
This is a Class I recall. A Class I recall is employed by the FDA when the device being recalled presents a significant risk of serious injury or death to the affected individuals. This is, of course, not the first recall of a medical device for this reason, nor will it be the last. Artificial hips, hernia meshes, vaginal meshes and other products have all been recalled recently because of the fact that they present serious health risks to the people in whom they are implanted.
Defective Medical Device Attorney
Defective medical devices constitute some of the worst-case scenarios where defective products are concerned. Remedying the situation may require a painful and dangerous surgery and, in some cases, the damage has already been done, even if the device is removed after the fact. If you were affected by a dangerous medical device and want to seek compensation in a civil court, contact a defective medical devices attorney who specializes in these types of claims. They are sometimes handled through class action lawsuits and are sometimes handled on a one by one basis. No matter how your claim is handled, it may end up winning you substantial compensation.